Biosimilars are well established and approved following the same effectiveness, safety, and quality standards as reference biologics - based on the entirety of the data from a thorough comparability exercise. However, certain information gaps still exist about biosimilarity concepts and the therapeutic use of biosimilars (learn more in the Concepts of biosimilars section). To close these gaps, medical education is crucial for facilitating collaborative and informed clinical decision-making, to further improve patient outcomes and treatment compliance. 1
Across all therapeutic areas, healthcare professionals (HCPs) aim to optimize patient treatment to improve their quality of life. A good understanding of the benefits of biosimilars therapy and its potential to optimize treatment regimens will support in addressing unmet medical needs and improve patient clinical outcomes. During the treatment process, multidisciplinary teams (MDTs), comprising of HCPs with different specialties (physicians, nurses, pharmacists, therapists, social workers), provide a good patient support, communication and help enhance treatment efficiency. 2,3 Therefore, ongoing communication is crucial to a well-informed dialogue that can help patients with challenges and concerns during the treatment course.
Shared decision-making and patient education are central when using biosimilars 4
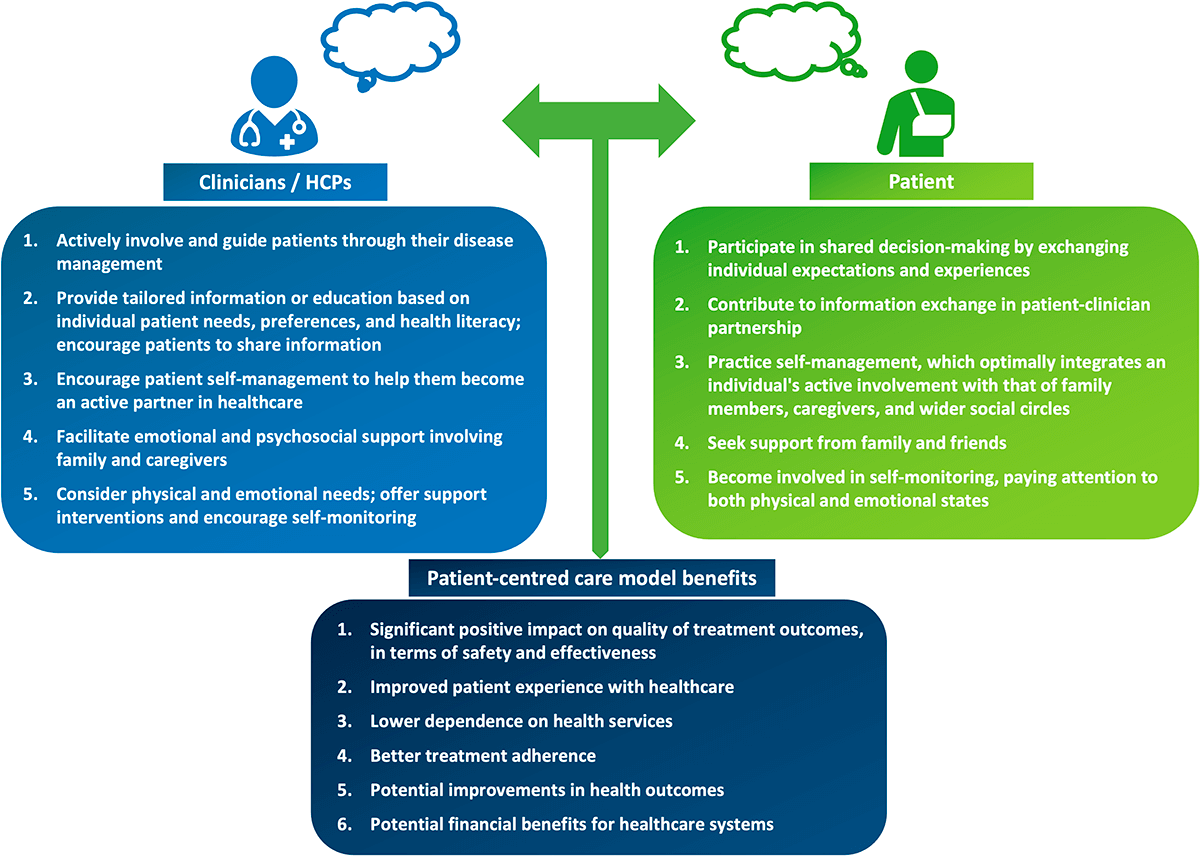

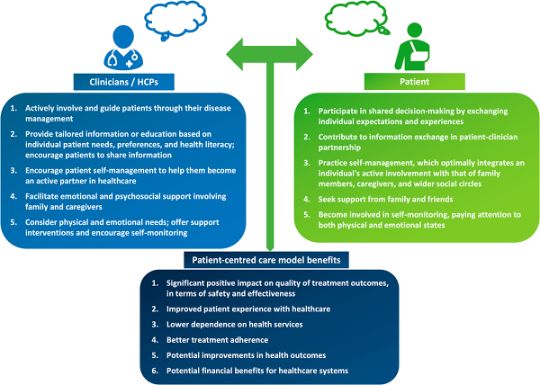
Figure adapted from Voshaar MJ, et al. Best Pract Res Clin Rheumatol 2015;29(4-5):643–663.
Switch
Advanced biologic medicines are now widely used to help treat patients with chronic debilitating and life-threatening diseases. 1 However, the positive impact of biologic therapies is often restricted through limited access and associated with high costs. 5 Use of biosimilars, being more affordable biologics compared to their reference products, can lead to increased accessibility and earlier treatment. The focus should be on the best process to switch in a shared decision-making model. 6
Whilst most HCPs are aware of biosimilars, certain knowledge gaps in understanding regarding their safety profiles and effectiveness can remain, which may cause uncertainty when using them in daily clinical practice. Physician and patient awareness, knowledge, and perceptions can impact the use of biosimilars and clinical outcomes. Patients that are aware and well-informed about biosimilars concepts and their use in clinical practice, often feel more positive about their treatment. In contrast, limited awareness and lingering misconceptions, whether among clinicians or patients, can foster uncertainty, making individuals less comfortable starting or switching to biosimilars and, in turn, potentially affecting treatment outcomes. 1,7,8
Biosimilars: switching vs substitution



A common phenomenon known as nocebo effect
Several studies have revealed non-adherence to prescribed therapies in a proportion of patients with immuno-mediated inflammatory diseases (IMIDs) including psoriasis, rheumatoid arthritis (RA) and inflammatory bowel disease (IBD). 12 A number of factors have been associated with non-adherence, including ‘non-specific side effects’, which have been attributed to the so-called nocebo effect. 13
Contrary to the placebo effect – the effect of the patients’ positive expectations related to their state of health – the nocebo effect represents the result of negative expectations and is an outcome of complex interactions between the patient, their surrounding general psychosocial situation, the HCP, and the way information is delivered and received. 14,15
The nocebo effect: the negative equivalent of placebo
- Placebo effect: beneficial outcomes provoked by sham therapy 15
- Nocebo effect: the induction or worsening of symptoms induced by sham or active therapies 15



Adapted from Colloca L & Barsky AJ. N Eng J Med 2020;382:554–561
Nocebo responses, which can contribute to the onset or worsening of symptoms, may be linked to perception gaps and have been observed in patients switching from reference biologics to biosimilars. This effect has been associated with slightly higher discontinuation rates compared to those seen with the reference therapy. 1,16 As nonadherence can be driven by the nocebo effect, with significant consequences for patients and healthcare systems, it is recommended that at treatment initiation HCPs clearly explain potential side effects and complications and engage in open patient-physician discussion to help mitigate nocebo-related impacts and support informed decision-making. 1,16
What HCPs need to be aware of regarding the nocebo effect 17
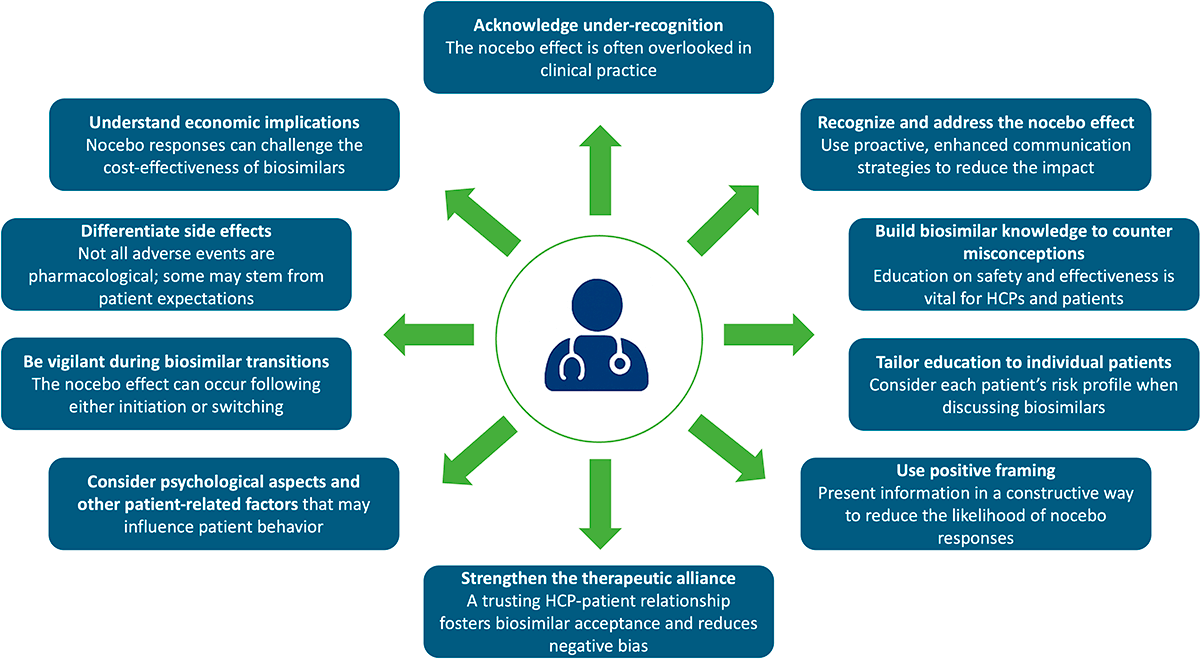

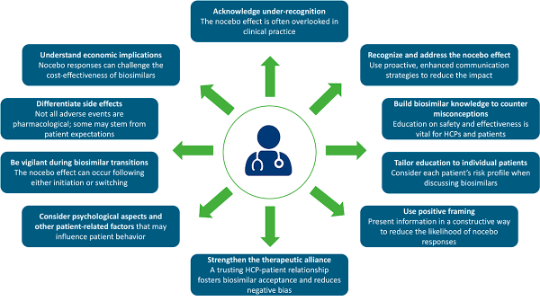
Adapted from Pouillon L, et al. Aliment Pharmacol Ther 2019;49:1181–1187.
Given the important role of biosimilars in improving patient access to effective biologic medicines with established safety profiles, it is important that HCPs are aware about the nocebo response as this may occur when patients are switched from a reference biologic to the biosimilar. HCP patient communication is crucial in addressing patient concerns and bridging knowledge gaps to promote optimal adherence. They can build trust and enhance treatment outcomes by assisting patients in understanding biosimilars as an effective and feasible therapeutic option, improving their perception through well-informed and confident communication. 1
Educating about switch and nocebo effect quick cards
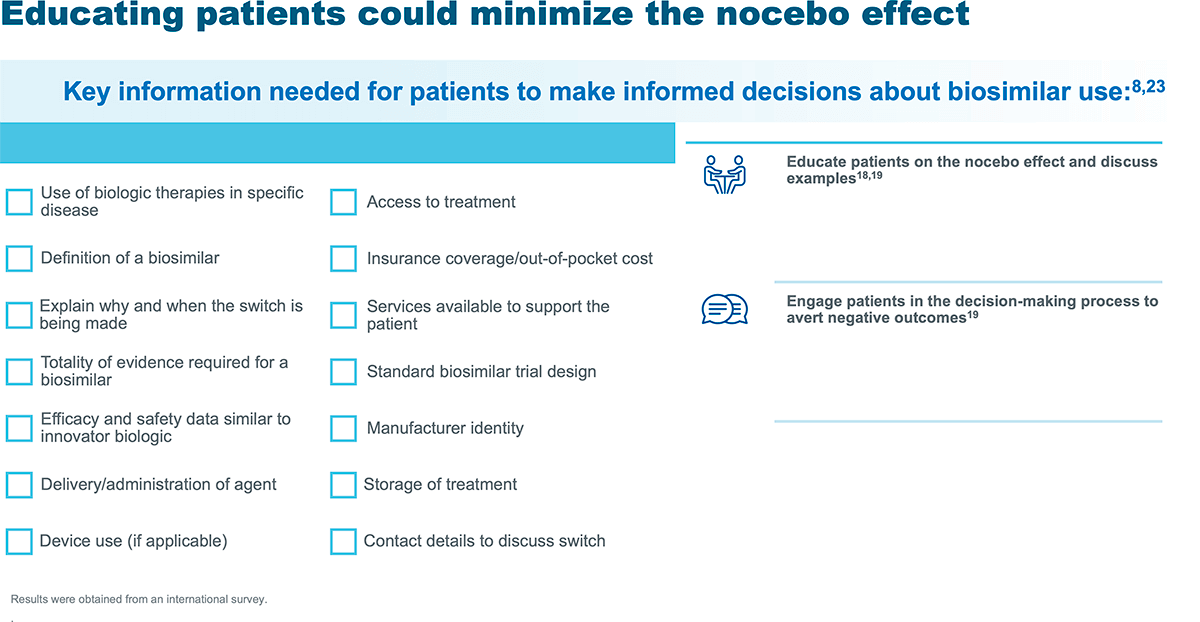
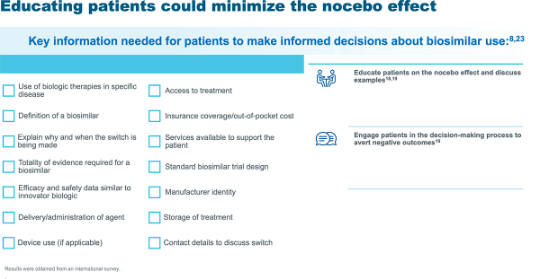
See below some suggestions about communication strategies for HCPs to educate patients about switching to a biosimilar and nocebo effect, and a checklist for HCPs with useful information for patients to make informed decisions about biosimilars use10:



*More side effects are reported by patients who use the internet. †Costs or pressure from health insurance or representatives. ‡Author opinion. Biogen recommends to always adequately inform patients of benefits and risks of their treatments. Positive framing of AEs may contribute to reducing nocebo effects (Webster RK, et al. Ann Behav Med 2018;52:920–29).
Adapted from Taylor P, et al. EMJ Rheumatol 2018;5:36–43.




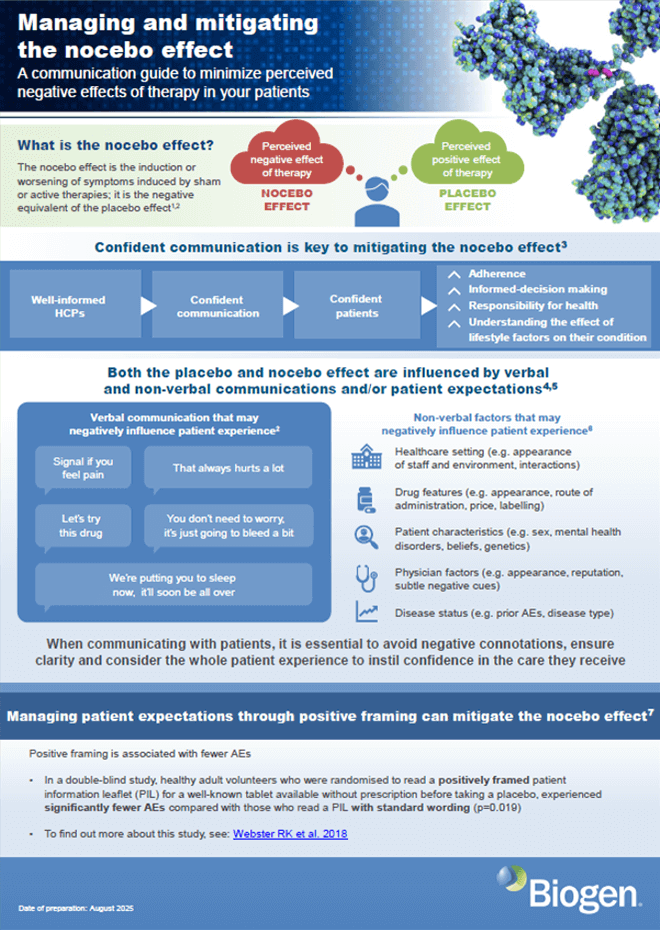
Nocebo flyer: communicating about the nocebo effect





Educational materials
Learn more about the key biosimilars concepts, switching, and the nocebo effect through the Basics of Biologics and Biosimilars Chapter 3 and Chapter 4:
Watch the following video animations and discover more about integrating biosimilars into daily clinical practice, including information about the nocebo effect, and the clinical and societal benefits of biosimilars:
1.Rezk MF, Pieper B. Rheumatol Ther. 2017;4(2):209–218.
2.NHS England. Information sharing in Multidisciplinary teams (MDTs). https://transform.england.nhs.uk/information-governance/guidance/information-governance-guidance-support-multidisciplinary-teams-mdts/. Accessed August 2025.
3.Taberna M, et al. Front Oncol. 2020;20:10:85.
4.Voshaar MJ, et al. Best Pract Res Clin Rheumatol. 2015;29(4-5):643–663.
5.Dutta B, et al. BioDrugs. 2020;3(2)4:159–170.
6.GaBI Online. Comparison of the cost of development of biologicals and biosimilars. https://gabionline.net/reports/comparison-of-the-cost-of-development-of-biologicals-and-biosimilars. Accessed August 2025.
7.Cohen H, et al. Adv Ther. 2017;33(12):2160–2172.
8.Jacobs I, et al. Patient Prefer Adherence. 2016 May 26;10:937–948.
9.Druedahl LC, et al. PLoS One. 2022;17(1):e0262537.
10.Taylor P, et al. EMJ Rheumatol. 2018; 5:36–43.
11.Webster RK, et al. Ann Behav Med. 2018;52(11):920–929.
12.Michetti P, et al. Adv Ther. 2017;34(9):91–108.
13.Barsky AJ, et al. JAMA. 2002;287(5):622–627.
14.Colloca L, Barsky AJ. N Engl J Med. 2020;382(6):554–561.
15.Häuser W, et al. Dtsch Arztebl Int. 2012;109(26):459–465.
16.Rezk MF, Pieper B. Adv Ther. 2018;3(6)5:749–753.
17.Pouillon L, et al. Aliment Pharmacol Ther. 2019;49(9):1181–1187.
18.Planès S, et al. Pharmacol Res Perspect. 2016;4(2):e00208.
19.Kristensen LE, et al. BioDrugs. 2018;3(5)2:397–404.
20.Daniali H, Flaten MA. Front Psychiatry. 2019 April 15;10:242.
21.Colloca L, Finniss D. JAMA. 2012;307(6):567–568.
22.Enck P, et al. Nat Rev Drug Discov. 2013;12(3):191–204.
23.Edwards C, et al. EMJ Rheumatol. 2017:42–48.
24.Afzali A, et al. Adv Ther 2021;38(5):2077–2093.
Abbreviations:
Learn more
Read more about the nocebo effect and treatment outcomes with Biosimilars in the following publications:
Rezk MF and Pieper B, 2018, Adv Ther; 35(6):749-753.
To See or NOsee: The Debate on the Nocebo Effect and Optimizing the Use of Biosimilars.
The authors take a closer look at the negative impact of the nocebo effect, as reported in a number of clinical trials, and its implications on patients’ perception when switching from an originator biologic to a biosimilar.
Rezk MF and Pieper B, 2017, Rheumatol Ther; 4(2):209-218.
Treatment Outcomes with Biosimilars: Be Aware of the Nocebo Effect.
The authors focus on the wide adoption of biosimilars, the reemergence of the nocebo effect and the implications it may have on both patients and physicians’ perceptions as well as on treatment success. The authors also provide practical strategies and recommendations to raise awareness and to limit the nocebo effect.







